Korean Medical Device Approval Timeline
Medical devices are classified into four categories, and the time required for approval varies depending on the class.
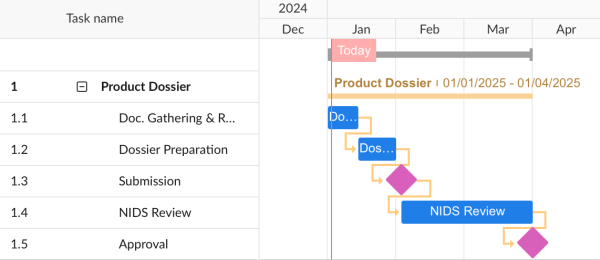
Class 1
- Class 1 medical devices do not require submission of KGMP certification or product testing data.
- Class 1 medical devices typically take 2-3 months for approval (registration).
- The following timeline takes into account first and second revisions.
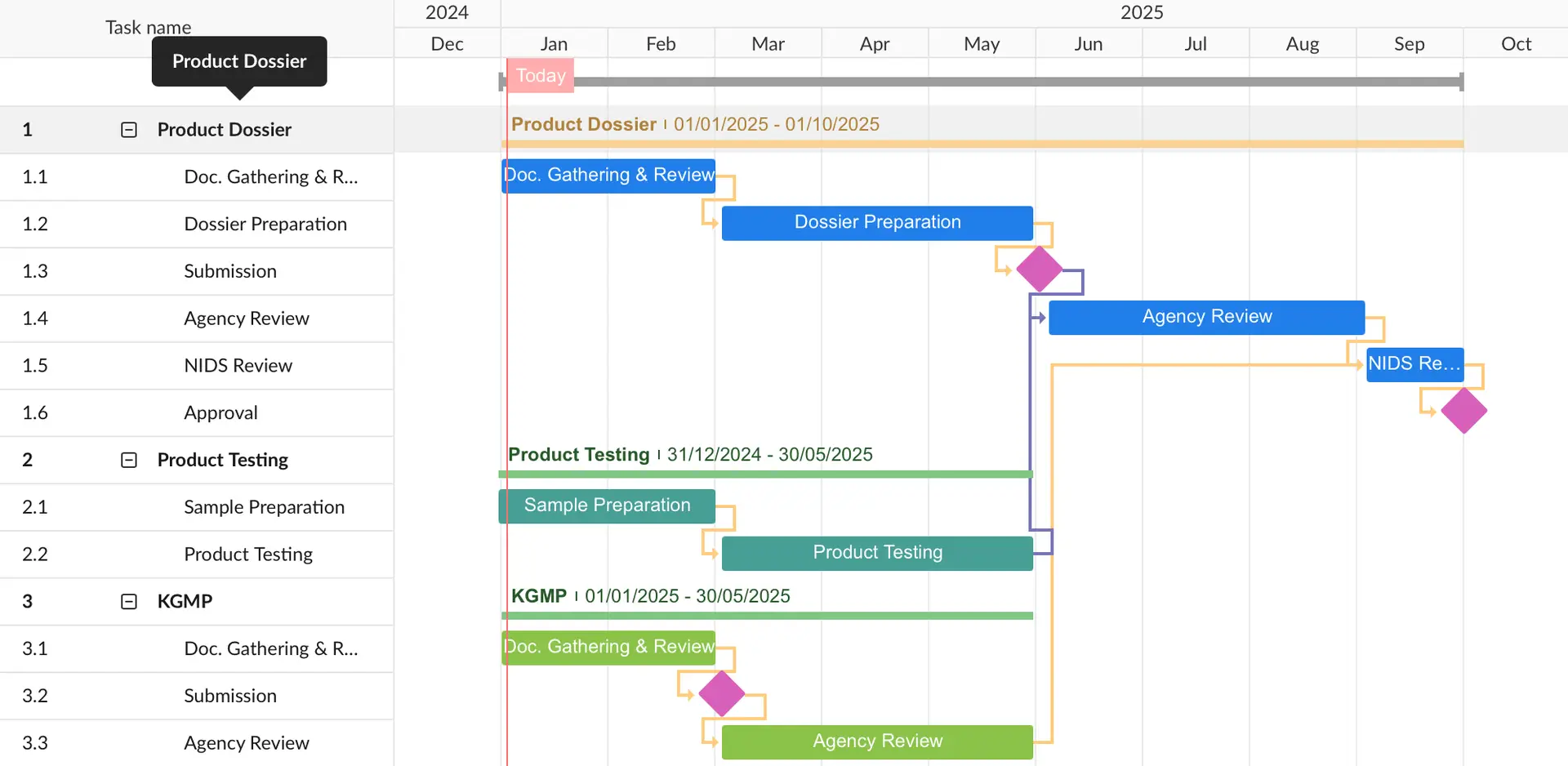
Class 2, 3, 4:
- The approval time varies depending on whether product testing is conducted in Korea, whether KGMP local audits are required, and whether clinical data review is necessary.
- Class 2 devices generally take 6-9 months for approval (certification).
- Class 3 and 4 devices typically take 9-12 months for approval, as the review by the Korean MFDS takes more time.
- The following timeline assumes product testing for Class 2 devices, KGMP document review, and no clinical data requirement.